High yielding synthesis of 2,2′-bipyridine macrocycles, versatile intermediates in the synthesis of rotaxanes†
Abstract
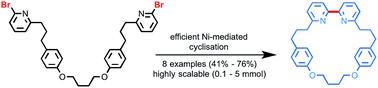
We present an operationally simple approach to 2,2′-bipyridine macrocycles. Our method uses simple starting materials to produce these previously hard to access rotaxane precursors in remarkable yields (typically >65%) across a range of scales (0.1–5 mmol). All of the macrocycles reported are efficiently converted (>90%) to rotaxanes under AT-CuAAC conditions. With the requisite macrocycles finally available in sufficient quantities, we further demonstrate their long term utility through the first gram-scale synthesis of an AT-CuAAC [2]rotaxane and extend this powerful methodology to produce novel Sauvage-type molecular shuttles.

 Please wait while we load your content...
Please wait while we load your content...