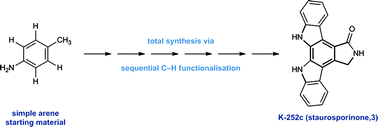
The total synthesis of K-252c (staurosporinone) via a sequential C–H functionalisation strategy†
Abstract
A synthesis of the bioactive indolocarbazole alkaloid K-252c (staurosporinone) via a sequential C–H functionalisation strategy is reported. The route exploits direct functionalisation reactions around a simple arene core and comprises of two highly-selective copper-catalysed C–H arylations, a copper-catalysed C–H amination and a palladium-catalysed C–H carbonylation, which build up the structural complexity of the natural product framework.
- This article is part of the themed collections: Celebrating our 2019 Prize and Award winners and Top 50 Articles of 2016: Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...