Stereoselective synthesis of protected l- and d-dideoxysugars and analogues via Prins cyclisations†
Abstract
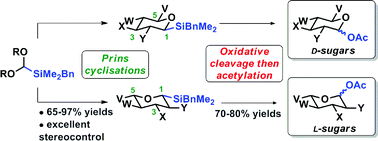
A de novo approach for the rapid construction of orthogonally protected L- and D-deoxysugars and analogues is described. A novel and robust silicon-acetal undergoes Prins cyclisations with a series of homoallylic alcohols in high yield and excellent stereocontrol. Modified Tamao–Fleming oxidation of the resulting silyltetrahydropyrans gives direct access to deoxyglycoside analogues and the approach was showcased in the synthesis of protected L-oliose, a component of the anticancer agent aclacinomycin A.

 Please wait while we load your content...
Please wait while we load your content...