Mechanism of the CuII-catalyzed benzylic oxygenation of (aryl)(heteroaryl)methanes with oxygen†
Abstract
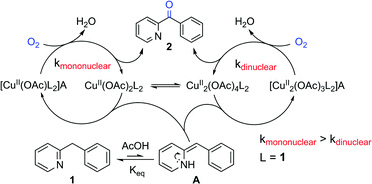
A mechanistic study of the copper-catalyzed oxidation of the methylene group of aryl(di)azinylmethanes was performed. Initial reaction rates were measured making use of in situ IR reaction monitoring and a kinetic analysis of the reaction was executed. The reaction proved to be first order in oxygen concentration. For substrate and acid concentration, saturation kinetics due to O2 mass transfer limitation were observed. The occurrence of mass transfer limitation was further confirmed by examining the effect of the stirring rate on the initial reaction rate. Interestingly, the effect of the concentration of the catalyst on the rate shows that higher loadings result in a maximal initial rate, followed initially by a steady decrease and subsequently a rate plateau when the concentration is increased further. Mass transfer limitation and increased concentration of dinuclear catalytically active species rationalizes this hitherto unprecedented rate behavior. Continuous-wave and pulsed electron paramagnetic resonance methods were used to characterize the catalytic species present in the solution during the reaction and confirmed the presence of both mono- and dinuclear copper species. Analysis of a diverse substrate scope points towards imine–enamine tautomerization as a crucial process in the oxidation reaction. DFT calculations of these equilibrium constants (pKeq) provided us with a qualitative tool to predict whether or not a substrate is viable for oxidation under the reaction conditions developed.


 Please wait while we load your content...
Please wait while we load your content...