Synthesis of open-shell ladder π-systems by catalytic C–H annulation of diarylacetylenes†
Abstract
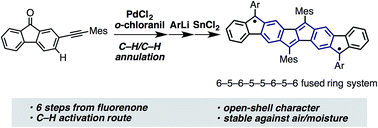
A new open-shell ladder-shaped π-system has been synthesized. Pentaleno[1,2-b:4,5-b′]difluorene derivatives, 8 fused ring systems bearing 5- and 6-membered rings, were constructed from alkynylfluorenone through a reaction sequence including Pd-catalyzed C–H/C–H annulation. X-ray crystallography and ESR spectroscopy revealed the open-shell character of these ladder-shaped molecules, which derives from their extended π-electron conjugation. Absorption peaks in the near IR region as well as narrow redox potentials observed by cyclic voltammetry indicated small optical and fundamental energy gaps of these fused ring systems.

 Please wait while we load your content...
Please wait while we load your content...