Chiral Brønsted acid-catalyzed enantioselective Friedel–Crafts reaction of 2-methoxyfuran with aliphatic ketimines generated in situ†
Abstract
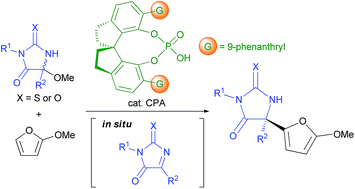
An enantioselective Friedel–Crafts reaction with aliphatic ketimines generated in situ from hemiaminal ethers catalyzed by a chiral Brønsted acid was investigated. The reaction of 2-methoxyfuran with (thio)hydantoin-derived hemiaminal methyl ether proceeded under the influence of a chiral phosphoric acid catalyst to afford the corresponding adduct possessing a quaternary stereogenic center in high yield with high enantioselectivity. Theoretical studies were also conducted to clarify the mechanism of the stereochemical outcome and the major factors contributing to the efficient enantioselection.

 Please wait while we load your content...
Please wait while we load your content...