Access to a new class of synthetic building blocks via trifluoromethoxylation of pyridines and pyrimidines†
Abstract
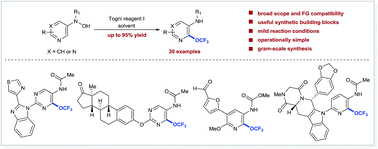
Since the first synthesis of trifluoromethyl ethers in 1935, the trifluoromethoxy (OCF3) group has made a remarkable impact in medicinal, agrochemical, and materials science research. However, our inability to facilely incorporate the OCF3 group into molecules, especially heteroaromatics, has limited its potential across a broad spectrum of technological applications. Herein, we report a scalable and operationally simple protocol for regioselective trifluoromethoxylation of a wide range of functionalized pyridines and pyrimidines under mild reaction conditions. The trifluoromethoxylated products are useful scaffolds that can be further elaborated by amidation and palladium-catalysed cross coupling reactions. Mechanistic studies suggest that a radical O-trifluoromethylation followed by the OCF3-migration reaction pathway is operable. Given the unique properties of the OCF3 group and the ubiquity of pyridine and pyrimidine in biologically active molecules and functional materials, trifluoromethoxylated pyridines and pyrimidines could serve as valuable building blocks for the discovery and development of new drugs, agrochemicals, and materials.
- This article is part of the themed collection: Top 50 Articles of 2016: Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...