Synthesis and biological evaluation of new 2,5-dimethylthiophene/furan based N-acetyl pyrazolines as selective topoisomerase II inhibitors†
Abstract
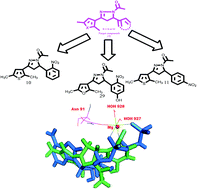
Based on reported pharmacophores as topoisomerase inhibitors, 2,5-dimethylthiophene/furan based N-acetyl pyrazolines were designed and envisaged as topoisomerase inhibitors. The target compounds were synthesized and tested in vitro against human topoisomerases in decatenation, relaxation, cleavage complex and DNA intercalation assays. Out of 29 compounds, three (10, 11 and 29) showed potent and selective topoisomerase II inhibitory activity with no intercalation with DNA. Further, molecular docking studies also endorsed them as ATP dependent topoisomerase II catalytic inhibitors. These compounds exerted potential anticancer effects on breast, colon, lung and prostate cancer cell lines at a low micromolar level, as compared to etoposide, and low toxicity to normal cells. Apart from the topoisomerase II inhibition, these compounds also induced a reactive oxygen species (ROS) level in cancer cells. The cell cycle analyses showed their apoptotic effect at the G1 phase.


 Please wait while we load your content...
Please wait while we load your content...