DNA and BSA binding, anticancer and antimicrobial properties of Co(ii), Co(ii/iii), Cu(ii) and Ag(i) complexes of arylhydrazones of barbituric acid†
Abstract
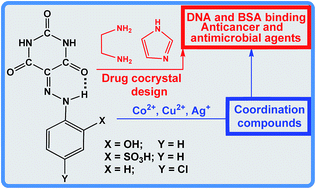
Two new cocrystalline compounds, (Hen)(H2L2)·2/3H2O (2) and (Him)(H3L3)·2H2O (8), were prepared by the reaction of 5-(2-(4-chlorophenyl)hydrazono)pyrimidine-2,4,6(1H,3H,5H)-trione (H3L2) and the sodium salt of 2-(2-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)hydrazinyl)benzenesulfonic acid (H4L3), [Na(H3L3)(μ-H2O)(H2O)2]2 (1) with protonated ethylenediamine (Hen) and imidazole (Him), respectively. By using 5-(2-(2-hydroxyphenyl)hydrazono)pyrimidine-2,4,6(1H,3H,5H)-trione (H4L1) and 1, several known CuII, CoII, CoII/III and new AgI complexes, [Cu(H2L1)(H2O)(im)]·3H2O (3), [Co(H2O)6][Co(H2L1)2]2·8H2O (4), [Co(H2L3)(im)3] (5), [Cu(H2L3)(im)2]·H2O (6), [Ag(H2O)(μ-H3L3)]n (7) and [Co(H2O)6][H3L3]2·8H2O (9), were also prepared in order to study their DNA and BSA binding, anticancer and antimicrobial properties. The complexes are able to interact with DNA and BSA with high binding constant values. In particular, complex 4 strongly intercalates DNA and binds BSA. The antimicrobial activity of all compounds, determined against a panel of reference bacterial and fungal strains, indicates that only 2 and 7 possess antimicrobial activity. The same compounds 2 and 7 show a pronounced antiproliferative activity against the human tumor cell lines A375, MDA-MB 231 and HCT116.

 Please wait while we load your content...
Please wait while we load your content...