Synthesis, characterization and biological activity of fluorescently labeled bedaquiline analogues†
Abstract
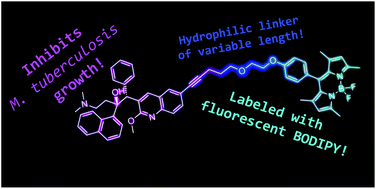
Diarylquinolines represent a new class of antibiotics with high potency against Mycobacterium tuberculosis. As such, they are of utmost importance in the treatment of drug-resistant bacterial pathogens. In this work, we report a strategy for preparing fluorescently labeled derivatives of the FDA-approved diarylquinoline-based tuberculosis drug bedaquiline. The labeled compounds were capable of blocking bacterial growth and interfered with the function of ATP synthase, the cellular target of diarylquinolines. This indicates that the chosen labeling strategy does not preclude the antibacterial activity of bedaquiline, and allowed us to investigate the effect of labeling on drug recognition by bacterial efflux pumps in living M. tuberculosis strains. These properties, coupled with the efficient fluorescence of the attached BODIPY fluorophore means that these compounds can be used as a research tool to gain deeper understanding into the mechanism of action of this class of drugs.


 Please wait while we load your content...
Please wait while we load your content...