Synthesis, characterization and electrochemical evaluation of mixed oxides of nickel and cobalt from spent lithium-ion cells
Abstract
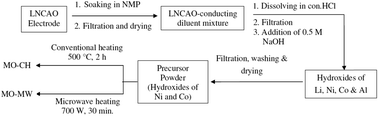
A simple and easy strategy for the synthesis of mixed oxides of Ni and Co from spent Li-ion cell cathodes based on LiNi0.8Co0.15Al0.05O2 (LNCAO) active material is presented here. The separation of the precursor of the active materials in the hydroxide form is achieved using a chemical precipitation technique and it is then subjected to heat treatment following two routes: (i) conventional heating, and (ii) microwave heating. The products thus obtained are evaluated as anode material in Li-ion cells using Li metal as the counter electrode. The mixed oxides of Ni and Co synthesised via the microwave route (MO-MW) perform better compared to those obtained following the conventional heating route (MO-CH). The MO-MW electrode shows an initial specific capacity of 1104 mA h g−1 and retains 846 mA h g−1 after 30 charge–discharge cycles, whereas, the MO-CH electrode delivers an initial specific capacity of 763 mA h g−1 with retention of 71% after 30 cycles. The metal oxide composite structure, its nano-dimensions as well as the spherical morphology of the MO-MW material contributed towards its better electrochemical performance.

 Please wait while we load your content...
Please wait while we load your content...