Ring opening polymerization of ε-caprolactone in the presence of wet β-cyclodextrin: effect of the operative pressure and of water molecules in the β-cyclodextrin cavity
Abstract
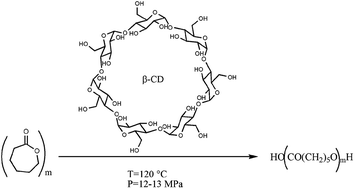
The ring opening polymerization (ROP) of ε-caprolactone (CL) in the presence of β-cyclodextrin (β-CD) was performed in batch reactors both at room pressure and with the reaction system pressurized with CO2, N2 or Ar. Significant enhancements of the polymerization rate was observed when the ROP was carried out with wet β-CD under pressure. For example, after 24 hours at 120 °C with a β-CD/CL molar ratio of about 1/100, the monomer conversion increased from 4 to 98–99% when the pressure was changed from 0.1 to 12.5–13.0 MPa independent of the nature of the compressing gas. MALDI-TOF analyses indicated that a major fraction of polymer chains obtained in pressurized systems was initiated by water molecules. The collected results suggest that at 12–13 MPa wet β-CD can catalyse both the ring opening of ε-caprolactone and the polymerization of the obtained linear species and that high energy water molecules located inside the cavity of the cyclic oligosaccharide must play a role in initiating the polymerization. The trend of number average molecular weight and the results of MALDI-TOF analyses obtained in polymerizations performed for long reaction times and in a hydrolysis test of commercial poly(ε-caprolactone) indicate that wet β-CD can work as an artificial lipase enzyme under the adopted conditions.

 Please wait while we load your content...
Please wait while we load your content...