{Co II/III5} horseshoe and {Ni II4} lacunary cubane coordination clusters: the isobutyrate/N-butyldiethanolamine reaction system†
Abstract
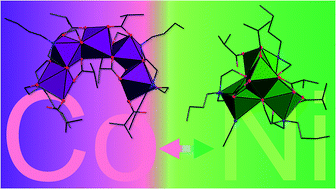
The polynuclear coordination compounds [CoII3CoIII2(Hbda)2(bda)2(ib)6]·2MeCN (1) and [NiII4(Hbda)3(ib)5(MeCN)] (2) (H2bda = N-butyldiethanolamine, ib = isobutyrate) are prepared under aerobic conditions using an identical synthetic protocol that solely differs in the employed transition metal (CoII vs. NiII). Whereas compound 1 displays a mixed-valent, pentanuclear, horseshoe-shaped structure with alternating Co(II) and Co(III) ions, compound 2 presents a tetrahedrally-shaped Ni(II) structural motif where four nickel centers are bridged by three O atoms to afford a lacunary Ni4O3 cubane, a motif hitherto only observed as a substructure of higher-nuclearity coordination clusters and polyoxometalates. Both compounds are thermally surprisingly stable (>130 °C). 1 exhibits weak antiferromagnetic exchange interactions; 2 shows a ferromagnetic coupled triangle of three Ni centers interacting antiferromagnetically with a single Ni apex.


 Please wait while we load your content...
Please wait while we load your content...