1,3-Dialkylimidazolium modified clay sorbents for perchlorate removal from water†
Abstract
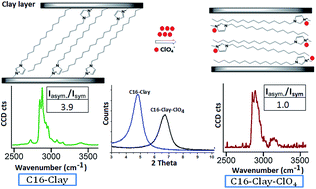
Sodium montmorillonite clays modified using 1-alkyl-3-methylimidazolium based ionic liquids with varying alkyl chain length viz. C4, C6, C8, C10 and C16 were used for perchlorate adsorption from water. Pristine MMT showed negligible adsorption whereas ionic liquid modified clays showed an increase in adsorption with increase in chain length of the exchanged cation. 1-Hexadecyl-3-methylimidazolium modified clay (C16-clay) with a d-spacing of 18.55 Å showed a maximum adsorption of 0.16 mmol g−1 of clay. The d-spacing of the C16-clay decreased on adsorption of perchlorate to 13.70 Å without a change in the composition of the modified clay, as confirmed by CHN analysis. Raman spectroscopic studies substantiated the conformational change from gauche to trans for the imidazolium cations on perchlorate adsorption. The adsorption followed the Freundlich isotherm with pseudo second order kinetics. The modified clays were thermally stable (<200 °C) and regenerated by heating to 175 ± 5 °C in air and 95% regenerability was observed.

 Please wait while we load your content...
Please wait while we load your content...