Low-temperature gas–solid carbonation of magnesia and magnesium hydroxide promoted by non-immersive contact with water†
Abstract
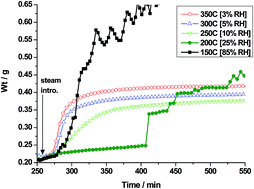
From gas–solid carbonation studies and product characterization by XRD, carbon elemental analysis, and TG-FTIR profiling of evolved CO2, the presence of water vapour at high relative humidity (>25% RH) was shown to cause a drastic acceleration in the rate of CO2 absorption into MgO and Mg(OH)2 producing magnesite and hydrocarbonated precursors. From thermogravimetric experiments in the vicinity of the dew point, carbonation was shown to proceeded in steps triggered by spontaneous condensation (and re-evaporation) of water. Almost complete conversion to magnesite (MgCO3) and/or hydromagnesite [HM = 4MgCO3·Mg(OH)2·4H2O] was observed in a few hours under rather mild conditions of pressure (PCO2 ≤ 10 bar) and temperature (T ≤ 150 °C). Unwashed (sulphate-contaminated) Mg(OH)2 extracted from serpentinite mineral via the Åbo Akademi sulphation/precipitation route yielded MgCO3 selectively. Washed extracts and commercial samples formed mainly HM. Carbonation was even measurable below 50 °C using sub-atmospheric pressures of CO2 typical of flue gas. The rate was quite insensitive to PCO2. Complete conversion to nesquehonite [MgCO3·3H2O] and/or dypingite [4MgCO3·Mg(OH)2·5H2O] was achieved by overnight treatment. High-pressure scale-up tests (1–35 g) on commercial Mg(OH)2 (Aldrich, >95%) from 70–150 °C under equilibrated batch conditions at 100% RH yielded HM in the 1st hour. At longer times, HM seemed to transform spontaneously into MgCO3. Judiciously pre-dampened samples were more reactive, re-affirming the importance of ambient water. Water as a promoter in the more speculative direct gas–solid mineral carbonation is briefly evaluated.

 Please wait while we load your content...
Please wait while we load your content...