Phase relation, structure and ionic conductivity of Li7−x−3yAlyLa3Zr2−xTaxO12†
Abstract
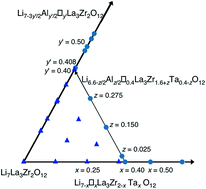
The phase relation, structure and ionic conductivity of garnet-like Li7−x−3yAlyLa3Zr2−xTaxO12 were investigated. The improved sample dissolution process in the ICP measurements enabled the exclusion of the ambiguity of the atomic composition in the samples. The tetragonal phase formed at x = 0–0.375 and the cubic phase appeared with x = 0.4–2.0 in the Li7−xLa3Zr2−xTaxO12 system. In the Al-doped system of Li7−x−3yAlyLa3Zr2−xTaxO12, the tetragonal phase was formed at x + 3y < 0.4. The border between the tetragonal and the cubic phases exists at Li6.6−z/2Alz/2□0.4La3Zr1.6+zTa0.4−zO12. The tetragonal/cubic structure change corresponds to the order/disorder of lithium ions and is dependent on the cation content at the lithium sites. The ionic conductivity of the cubic compounds has a positive tendency with respect to the lithium content, whereas that of the tetragonal compounds is opposite. A high total ionic conductivity exceeding 5.0 × 10−4 S cm−1 at 25 °C was observed for Al-doped Li6.6−z/2Alz/2La3Zr1.6+zTa0.4−zO12. The highest total conductivity of 1.03 × 10−3 S cm−1 at 25 °C with an activation energy of 0.35 eV was obtained at z = 0.275. Nuclear magnetic resonance spectroscopy revealed that Al3+ substitution decreases the diffusion of lithium ions in the structure. The high total conductivity of Al-doped Li6.6−z/2Alz/2La3Zr1.6+zTa0.4−zO12 may be due to the enhancement of lithium diffusion at the grain boundaries.

 Please wait while we load your content...
Please wait while we load your content...