Tandem Mannich/Diels–Alder reactions for the synthesis of indole compound libraries†
Abstract
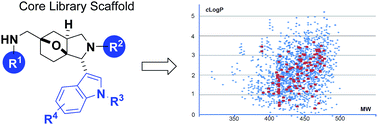
A tandem Mannich/Diels–Alder sequence for the synthesis of small-molecule libraries with an indolyl-octahydro-3a,6-epoxy-isoindole core structure is demonstrated in this study. Representative diversification examples based on this scaffold were performed, and a library is being produced within the European Lead Factory (ELF) Consortium.
- This article is part of the themed collection: Elegant Synthetic Routes to Indole Derivatives

 Please wait while we load your content...
Please wait while we load your content...