Monoclonal antibody capture from cell culture supernatants using epitope imprinted macroporous membranes
Abstract
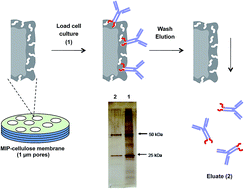
Epitope-imprinted membranes targeting the C-terminal fragment of the immunoglobuline G (IgG) heavy chain was developed and used for the purification of a commercial monoclonal antibody. The membranes exhibited strongly enhanced IgG affinity when compared with non-imprinted or IgG imprinted membranes reflected in binding selectivities in a protein mixture (IgG/HSA 1 : 10 w/w) of up to 40, and the elution of 95 to 100% pure IgG after washing. The dynamic binding capacity amounted to 3.9 mg mL−1 membrane volume with minor loss in performance upon repeated cleaning with alkali. The depletion of host cell proteins from a cell culture broth after production of anti-IL8 antibody using the best performing imprinted membrane under low-salt conditions reached 88% (0.7–1.2 log units) implying an effective removal of impurities from the cell culture supernatant.

 Please wait while we load your content...
Please wait while we load your content...