Ce(SO4)2-catalysed the highly diastereoselective synthesis of tetrahydroquinolines via an imino Diels Alder ABB′ type reaction and their in vivo toxicity and imaging in zebrafish embryos†
Abstract
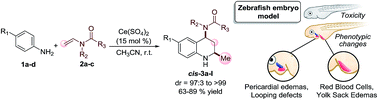
An efficient and practical approach has been developed for the synthesis of N-(tetrahydroquinolinyl-4) amides 3a–l in good yield with high diastereoselectivity. The strategy comprises the domino type ABB′ imino Diels Alder reaction catalysed by a cerium(IV) salt between anilines and N-vinyl amides for the preparation of a 12-membered library of tetrahydroquinolines that were tested for their in vivo toxicity against zebrafish embryos. Upon determining their LC50 values, N-(8-methoxy-2-methyl-tetrahydroquinolinyl-4) acetamide 3k was identified as the most toxic derivative with an LC50 below 95 μM (24 mg L−1). Finally, the phenotypes induced, at concentrations below their LC50, were analyzed at 48, 72 and 96 hours post fertilization, wherein the treated embryos manifested diverse visual phenotypes, such as big yolk sacs (3b, 3h, 3j), pericaldial edemas (3a, 3i) and red blood cells in the liver region (3b, 3l), in comparison to the morphology of the control embryos, the phenotypes could be associated with specific biological targets.

 Please wait while we load your content...
Please wait while we load your content...