Br2-Catalyzed regioselective dehydrative coupling of indoles with acyloins: direct synthesis of α-(3-indolyl) ketones†
Abstract
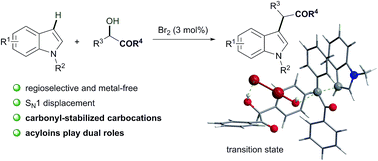
An efficient Br2-catalyzed synthesis of α-(3-indolyl) ketones via dehydrative coupling of simple indoles with acyloins is presented. This reaction proceeded with high C-3 selectivity and a wide substrate scope, and without any metal catalyst. Both the activation of alcohols by carbonyl groups and the catalysis of Br2 were essential. Density functional theory (DFT) calculations indicated that carbonyl groups not only enhanced the electrophilicities of the resulting carbocations, but acted as stabilizers for them as well, and that the activation of the catalyst by hydrogen bonding between Br2 and the hydroxyl hydrogen of acyloins was the key process for this transformation.

 Please wait while we load your content...
Please wait while we load your content...