Co-existence of amorphous and crystalline phases in Na-doped SrSiO3 system
Abstract
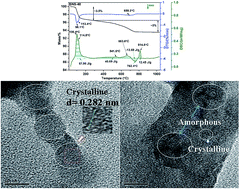
The exceptionally high ionic conductivity of Na-doped SrSiO3 solid electrolytes has attracted widespread attention for their use in SOFCs. The structural features are investigated using techniques such as X-ray diffraction (XRD), SEM/EDS, HR-TEM, DSC/TGA and Raman spectroscopy. The amorphous phase co-formed with the crystalline phase has been visualized using HR-TEM and co-related well with the XRD results. The glass transition (762 °C), glass melting (815 °C) and enthalpy of fusion (12.5 J g−1) of the amorphous phase are reported for the first time using DSC analysis. The thermal stability of the synthesized samples has also been studied using TGA analysis. Na doping in this system leads to the formation of a Na-rich glassy phase, which segregates along the grain-boundaries and is responsible for high electrical conductivity. Among all synthesized samples, Sr0.6Na0.4SiO3−δ showed the highest conductivity of 22.8 mS cm−1 at 800 °C in air. Also, strontium silicate forms the monoclinic phase and behaves as an insulator in the measured temperature range (200–800 °C).

 Please wait while we load your content...
Please wait while we load your content...