Synthesis, coordination behavior and structural features of chiral iron(ii) PNP diferrocene complexes†
Abstract
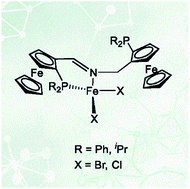
Five new chiral PNP ferrocene ligands with either an imine or amine nitrogen coordination site were synthesized. Only the imine type ligands formed Fe(II) complexes with the general formula [Fe(PNP)X2] (X = Cl, Br). In the solid state these complexes adopt a tetrahedral geometry with the PNP ligand coordinated in a κ2P,N-fashion with the one pendant-arm and the other not coordinated, as determined by X-ray crystallography and Mössbauer spectroscopy. The complexes are paramagnetic with a quintet ground state. In solution there is an equilibrium between [Fe(κ3P,N,P-PNP)X2] and [Fe(κ2P,N-PNP)X2] complexes. Boronation of the non-coordinated arm shifts the equilibrium towards the four-coordinate complex [Fe(κ2P,N-PNPBH3)Br2]. DFT calculations are consistent with the experimental results and indicate that the experimentally observed κ2 isomer is thermodynamically the most stable. In a CO atmosphere, [Fe(PNP)(CO)2Br]Br was formed rather than [Fe(PNP)(CO)Br2].

 Please wait while we load your content...
Please wait while we load your content...