Novel homologated-apio adenosine derivatives as A3 adenosine receptor agonists: design, synthesis and molecular docking studies†
Abstract
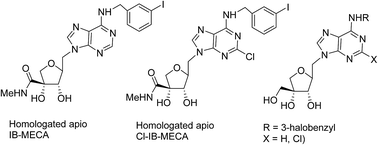
Synthesis of a series of novel homologated-apio adenosine analogues including homologated-apio IB-MECA and Cl-IB-MECA has been accomplished from a commercially available, inexpensive starting material D-ribose. The synthetic route includes a controlled oxidative cleavage of vicinal diol and Mitsunobu condensation reactions as the key steps. The molecular docking studies of the synthesized compounds to the A3 adenosine receptor model indicated them as agonists. Interestingly, some of the molecules showed good binding interactions at the active site as evident by docking scores and MM/GBSA binding affinity.

 Please wait while we load your content...
Please wait while we load your content...