Mechanistic outlook on thermal degradation of 1,3-dialkyl imidazolium ionic liquids and organoclays†
Abstract
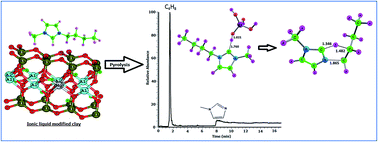
The thermal degradation mechanisms of ionic liquids (ILs) 1-butyl-3-methylimidazolium chloride (BMImCl) and 1-butyl-3-methylimidazolium tetrafluoroborate (BMImBF4) have been established using pyrolysis-GC-MS (Py-GC-MS) and B3LYP/6-311+G(d,p) level of density functional theory (DFT). BMImCl decompose through a bimolecular nucleophilic substitution (SN2) while BMImBF4 exhibit SN2 along with a competitive E2 elimination pathway. Activation energy parameters obtained using Kissinger–Akahira–Sunose method and Ozawa–Flynn–Wall method is compared with the computed activation barriers. The montmorillonite based organoclay prepared using these ionic liquids exchange only the cation part ([BMIm]+) into the clay gallery leading to an expansion of d-spacing from 12.08 to 13.64 Å. The organoclay showed the maximum decomposition at 462 °C in the TGA experiment and the decomposition products were identified as methyl imidazole and 1-butene using Py-GC-MS. DFT studies employing a model compound Si(OH)3O− suggested a mechanism involving an imidazole-2-ylidine (carbene) intermediate for the decomposition of [BMIm]+ in the clay. Theoretical results were further supported by 13C NMR analysis of IL in presence of colloidal silica which showed a characteristic carbene NMR signal at 187.6 ppm.

 Please wait while we load your content...
Please wait while we load your content...