Efficient conversion of glucose to 5-hydroxymethylfurfural using bifunctional partially hydroxylated AlF3†
Abstract
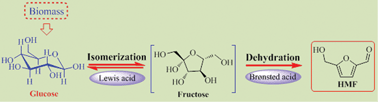
Partially hydroxylated AlF3 (denoted as AlF3) was synthesized by using a sol–gel method, and unambiguously characterized by FT-IR, XRD, NH3-TPD, SEM, TEM, N2 adsorption–desorption, and FTIR (pyridine-adsorption) techniques. The resulting mesoporous material (AlF3) simultaneously bearing Lewis and Brønsted acid sites was efficient for producing 5-hydroxymethylfurfural (HMF) from glucose, and an optimized HMF yield of 57.3% at a glucose conversion of 95.5% was obtained within 10 h at 140 °C. The effects of reaction temperature, time, catalyst amount, solvent and substrate type, and the amount of Brønsted/Lewis acid sites on conversion of glucose to HMF were also investigated. The existence of both Lewis and Brønsted acid sites in AlF3 was found to play a key role in glucose-to-HMF conversion.

 Please wait while we load your content...
Please wait while we load your content...