Effect of amino group on the excited-state intramolecular proton transfer (ESIPT) mechanisms of 2-(2′-hydroxyphenyl)benzoxazole and its amino derivatives
Abstract
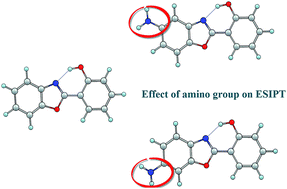
The excited-state intramolecular proton transfer (ESIPT) reactions of 2-(2′-hydroxyphenyl)benzoxazole (HBO), 5-amino-2-(2′-hydroxyphenyl)benzoxazole (5A-HBO) and 6-amino-2-(2′-hydroxyphenyl)benzoxazole (6A-HBO) were investigated with the time-dependent density functional theory (TD-DFT) method at the B3LYP/6-31G(d,p) theoretical level. The primary bond lengths and infrared (IR) vibrational spectra show that the intramolecular hydrogen bond is significantly strengthened in S1 state. The Mulliken's charge distribution and the frontier molecular orbitals (MOs) were analyzed. The result is consistent with the ESIPT mechanism proposed by Han and co-workers. Upon photo-excitation, the intramolecular hydrogen bond of 5A-HBO-enol (1.73 Å) and 6A-HBO-enol (1.74 Å) in the S1 state is weaker than that of HBO-enol (1.69 Å) due to the influence of the amino group in the HBO framework. After vertical excitation to the S1 state, the electronic density redistributes and migrates from the phenol ring to the benzoxazole ring of HBO. While for 5A-HBO and 6A-HBO, it transfers from the amino-benzoxazole moiety to the phenol ring. The analysis of the potential energy curves of HBO, 5A-HBO and 6A-HBO indicates that the ESIPT process of HBO occurs most easily. It is demonstrated that the presence and the position of the amino group in the HBO framework can change the behavior of the intramolecular hydrogen bonds O–H⋯N in the S1 state and thus hinder the ESIPT processes to some extent.

 Please wait while we load your content...
Please wait while we load your content...