Metal ion-immobilized magnetic nanoparticles for global enrichment and identification of phosphopeptides by mass spectrometry†
Abstract
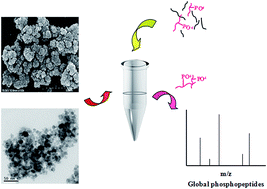
Phosphopeptide enrichment is critical for the deep analysis of the phosphoproteome, and many strategies have been developed to improve the coverage of identified phosphopeptides. However, the isolation of multiple phosphorylated peptides is still limited due to their distinct affinity capability to various materials. For example, titanium dioxide is preferred to isolate mono-phosphopeptides owing to its very strong binding of multiple phosphorylated peptides. Here, a new set of different metal ion (Ti4+, Zr4+, Fe3+, Tb3+, Tm3+, Ho3+)-immobilized magnetic nanoparticles, Fe3O4@TCPP-DOTA-Ms, were prepared, which possess the advantages of a stable metal complex, a large binding capacity and easy manual operation by introducing TCPP (tetrakis(4-carboxylphenyl) porphyrin), DOTA (1,4,7,10-tetraazacyclododecane N,N′,N′′,N′′′-tetraacetic acid) and various metal ions onto the magnetic nanoparticles. For the model protein α-casein tryptic digest, 15 phosphopeptides were identified with Fe3O4@TCPP-DOTA-Tb or Ti, and 46.67% of the enriched phosphopeptides were multiple phosphorylated peptides, indicating that the metal ion Tb3+ and Ti4+-immobilized materials have excellent enrichment efficiency and stronger adsorption for multiple phosphorylated peptides than other metal ion immobilized magnetic nanoparticles. Even in the tryptic digest of α-casein and BSA (1 : 50), 14–15 phosphopeptides were easily detected with Fe3O4@TCPP-DOTA-Tb/Ti, revealing that the novel materials possess strong selectivity for phosphopeptide enrichment. Additionally, Fe3O4@TCPP-DOTA-Tb/Ti was utilized to capture phosphopeptides in the tryptic digest of an extract of HeLa cells. In total, 13 450 phosphopeptides corresponding to 2965 phosphoproteins were identified in mass spectrometric analyses. The specificity for phosphopeptide enrichment was as high as 94%. More than half of the identified unique phosphopeptides were multiple phosphorylated peptides, which was much higher than that identified by the DHB/TiO2 (13.39%) method, making these materials a good choice for highly selective and global phosphopeptide enrichment in phosphoproteome analysis.

 Please wait while we load your content...
Please wait while we load your content...