Aromatic C–H bond cleavage by using a Cu(i) ate-complex†
Abstract
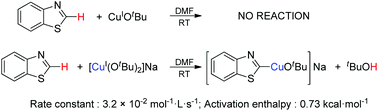
In situ X-ray absorption spectroscopy (XAS), infrared (IR) and nuclear magnetic resonance (NMR) techniques were used to identify the structures and reactivity of copper-containing active intermediates in the sp2 C–H bond cleavage reaction of electron-deficient aromatics. An ate-complex [Cu(OtBu)2]Na was found to be able to cleave the C–H bond of benzothiazole (ArH) producing [ArCuI(OtBu)]Na with a rate constant of 3.2 × 10−2 mol−1 L s−1 at −50 °C and with an activation enthalpy of 0.73 kcal mol−1 at room temperature.


 Please wait while we load your content...
Please wait while we load your content...