Low temperature dehydrations of non-activated alcohols via halide catalysis†
Abstract
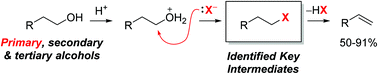
Activating kinetically inert C–O bonds such as in primary alcohols is an important challenge for the transformation of biomass-derived feedstocks. The herein described methodology addresses this issue through a combination of halide and acid catalysis. The novel mechanistic pathway proposed based on detailed experimental studies enables selective olefin formation from alcohols – as opposed to ether formation – at relatively low temperatures. Suitable substrates are tertiary, secondary, and even primary alcohols. Furthermore, the observed selectivity for the Hoffman elimination product and the realization of a non-rearranging Friedel–Crafts alkylation suggest that the reaction medium with high concentrations of halide (NBu4Br) enables reaction outcomes that cannot be obtained through carbocation intermediates.

 Please wait while we load your content...
Please wait while we load your content...