Heterocycle-derived β-S-enals as bifunctional linchpins for the catalytic synthesis of saturated heterocycles†
Abstract
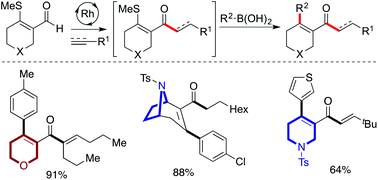
We demonstrate how heterocycle-derived β-S-enals can be employed as bifunctional substrates in a cascade of two rhodium-catalysed C–C bond forming reactions to deliver substituted heterocyclic products. A single rhodium-catalyst, generated in situ from a commercial salt and ligand combination, is used to promote both an initial alkene or alkyne hydroacylation reaction, and then a Suzuki-type cross-coupling, resulting in a three-component assembly of the targeted heterocycles. Substrates based on N-, O- and S-heterocycles are included, as are a range of alkenes, alkynes and boronic acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...