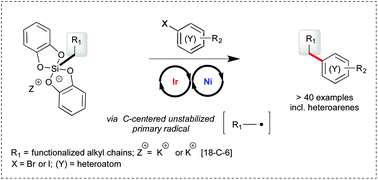
Primary alkyl bis-catecholato silicates in dual photoredox/nickel catalysis: aryl- and heteroaryl-alkyl cross coupling reactions†‡
Abstract
Primary alkyl bis-catecholato silicates have been successfully engaged with aryl and heteroaryl bromide substrates in photoredox/nickel dual catalysis to provide aryl- and heteroaryl-alkyl cross coupling products. The scope of the transformation is wide and the process appears to be tolerant of various functional groups present. Of note, most examples rely on the challenging use of highly reactive primary radicals which constitutes a significant advance in these cross coupling reactions.

 Please wait while we load your content...
Please wait while we load your content...