Post-polymerization modification of the side chain in optically active polymers by thiol–ene reaction†
Abstract
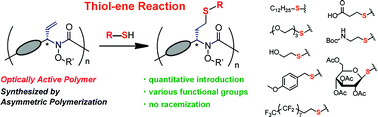
In this study, we examined novel synthetic methods for optically active polymers bearing various side chains from post-polymerization modification of a single optically active polymer. The side chain in the optically active polymer (poly-1) which was synthesized by asymmetric polymerization is modified using the thiol–ene reaction without racemization of the main chain. This transformation enables quantitative introduction of a wide range of functional groups, and the solubility of each resulting optically active polymer (poly-2) can be changed depending on the introduced substituent. The CD spectra of the modified polymer revealed the formation of a dynamic secondary structure through a new Cotton effect around 270 nm. In the experiments, the introduction of a dodecylthio group into the side chain restricted the flexibility of the main chain and suppressed the conformational fluctuations in the polymer to some extent. Moreover, when a hydroxyl group was introduced into the side chain, the Cotton effect was decreased, with the degree of intensity dependent on the solvent. Thus, the introduced substituents and polarity of the solvents influence the conformational fluctuations of the polymer.


 Please wait while we load your content...
Please wait while we load your content...