Transition-metal-free controlled polymerization of 2-polyfluorophenyl-5-trimethylsilylthiophenes: the substituent impact of fluorine†
Abstract
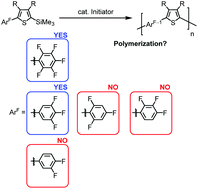
The design and synthesis of asymmetric AB-type monomers, 2-polyfluorophenyl-5-trimethylsilylthiophenes, and the transition-metal-free polymerization promoted by fluoride anions are described. The polymerization depended on the substitution position and also the number of fluorines on the phenyl groups; i.e., a 3,4,5-trifluorophenyl-substituted monomer proceeded the polymerization by a catalytic amount of potassium t-butoxide in the presence of cryptand[2.2.2] with controlled molecular weights and relatively low polydispersity indexes, but 2,4,6- and 2,3,4-trifluoro-substituted and also 3,4-difluoro-substituted monomers did not. The NMR spectra of the resulting polymers were consistent with a highly regulated structure for the polymer main chain, indicating that the polymerization process itself must be highly regioselective under these conditions. By using the polymerization, end-capping and block copolymerization with high fidelity were also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...