Direct arylation of fluoroarenes toward linear, bent-shaped and branched π-conjugated polymers: polycondensation post-polymerization approaches†
Abstract
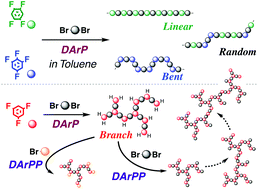
A direct arylation polycondensation of fluoroarenes with 2,7-dibromo-9,9-dioctylfluorene, F8, was achieved in a low polar solvent, toluene. The polycondensation of 1,2,4,5-tetrafluorobenzene, M1, or 2,2′,3,3′,4,4′,5,5′-octafluorobiphenyl, M1′, with F8 in toluene under our optimal conditions (2.5 mol% of palladium acetate, 5.0 mol% of tBu2MeHBF4, 2.0 equiv. of acetic acid, 3.0 equiv. of K2CO3, 0.6 M, 120 °C, 24 h) gave high molecular weight linear π-conjugated polymers (Mn > 60 000 and 40 000, respectively). Carboxylate additives promoted the direct arylation polycondensation. On the other hand, the direct arylation polycondensation of M1 with F8 gave insoluble products under the same conditions when DMAc was used instead of toluene. The network structure was probably formed by side reactions on the polymer chain. Thus, toluene is a useful solvent for the direct arylation polycondensation of fluoroarenes. In contrast with the linear (through) conjugated polymer synthesis, the direct arylation polycondensation of 1,2,3,5-tetrafluorobenzene, M2, with F8 gave bent-shaped (cross) conjugated polymers (Mn = 7300). The direct arylation polycondensation of 1,3,5-trifluorobenzene, M3 with F8 (monomer ratio 1 : 1) gave branched polymers having many unreacted C–H of trifluorobenzene inner and end units (Mn = 2900, DB = 53%). The degree of branching, DB, of the polymer was 53%, but the Mn value of the polymer was low (2900). Ozawa's conditions in toluene were also effective for synthesizing a high molecular weight linear polymer from F8 and M1. In contrast with our polycondensation, however, the polycondensation of F8 with M2 or M3 did not proceed well under Ozawa's conditions. Our polycondensation method is applicable to the synthesis of various fluoroarene-based polymers. The direct arylation post-polymerization (polymer reaction) to the remaining C–H bonds of the low-molecular weight branched polymer with F8 afforded a branched π-conjugated polymer with a higher molecular weight (Mn = 10 600, DB = 50%). This is the first example of direct arylation post-polymerization between “polymer and polymer” or “polymer and bromoarenes”.

 Please wait while we load your content...
Please wait while we load your content...