ADMET and TAD chemistry: a sustainable alliance†
Abstract
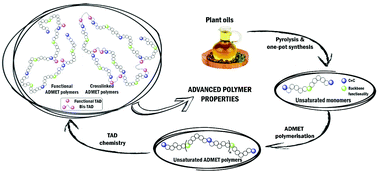
A versatile, additive-free post-polymerisation functionalisation method for acyclic diene metathesis (ADMET) derived polymers, utilising 1,2,4-triazoline-3,5-dione (TAD) chemistry, is reported. Several diene monomers have been synthesised starting from 10-undecenoic acid derivatives and subsequently polymerised via ADMET polymerisation reaction. Post-polymerisation functionalisation of the poly-unsaturated macromolecules with several substituted TAD compounds was shown to achieve full conversions within 6 hours. Also, the functionalised polymers could be chemically crosslinked with a bivalent triazolinedione crosslinker. The glass transition temperature of the resulting materials could be tuned by varying the degree of functionalisation, the polymer backbone, the TAD substituent and the crosslinking density.

 Please wait while we load your content...
Please wait while we load your content...