Synthesis of end-functionalized hydrogen-bonding poly(lactic acid)s and preferential stereocomplex crystallization of their enantiomeric blends†
Abstract
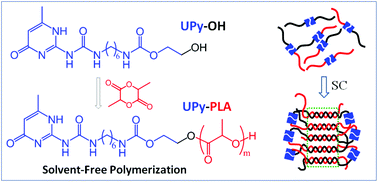
The solvent-free ring-opening polymerization (ROP) of lactide using the self-complementary quadruple hydrogen bonding 2-ureido-4[1H]-pyrimidinone (UPy)-functionalized alcohol as the initiator was achieved to attain UPy mono-functionalized poly(L-lactic acid) (PLLA) and poly(D-lactic acid) (PDLA) capable of undergoing supramolecular self-assembly. This ROP polymerization exhibits good controllability and the synthesized polymers have controlled molecular weights and well-defined terminal structures. The specific viscosities of UPy-functionalized PLLA and PDLA in dilute solution show strong concentration dependence, demonstrating the formation of a supramolecular structure by UPy dimerization. The crystallization kinetics, polymorphic crystalline structure, and crystalline structural organization of UPy-functionalized PLLA/PDLA blends were investigated and compared to the corresponding non-functionalized blends. The UPy end functionalization not only accelerates the crystallization but also facilitates the formation of high-melting-point stereocomplexes (SCs) in the PLLA/PDLA blends. The stereocomplexation ability of UPy-functionalized PLLA/PDLA blends further enhances on decreasing the molecular weights of PLLA and PDLA, and increasing the content of UPy end functionality. The incorporation of UPy end functionality also promotes the melt recrystallization of homocrystallites (HCs) to SCs upon heating. It is proposed that the promoted SC formation of the UPy-functionalized PLLA/PDLA blend originates from the enhanced interactions between enantiomeric chains.

 Please wait while we load your content...
Please wait while we load your content...