Polyacrylamide backbones for polyvalent bioconjugates using “post-click” chemistry†
Abstract
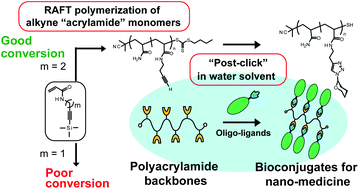
This paper reports the synthesis and application of acrylamide-type neoglycoconjugates interacting with practical targets. Polymer backbones with acrylamide and acrylamide derivatives bearing alkyne groups were prepared using living radical polymerization. Oligosaccharides were introduced into the backbones using copper-catalysed azide–alkyne cycloaddition “post-click” chemistry.


 Please wait while we load your content...
Please wait while we load your content...