Modular synthesis of glycopolymers with well-defined sugar units in the side chain via Ugi reaction and click chemistry: hetero vs. homo†
Abstract
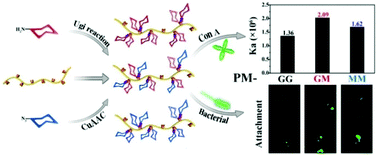
For synthetic glycopolymers, multivalent carbohydrate–protein interactions have been mostly studied in homoglycopolymers so far. However, natural oligosaccharides tend to consist of various sugars and this kind of heterogeneity is used to tune affinity and selectivity towards a specific receptor. Here we report a novel method to synthesize modularized heteroglycopolymers with well-defined sugar units in the side chain via Ugi reaction and click chemistry. To verify the modularized structures, mPEG-COOH was used as the model polymer substrate to test the Ugi reaction and subsequent click chemistry, and NMR, GPC and MALDI-TOF mass spectrometry confirmed our design. The obtained heteroglycopolymers (PM-GM) and homoglycopolymers (PM-GG and PM-MM) prepared via the methods assembled in aqueous medium with a diameter of 80 nm by DLS and 50 nm by TEM, and were used to investigate their interactions with lectins. Turbidity assay, QCM-D experiments and bacterial adhesion studies all demonstrate that heteroglycopolymers have higher affinity towards specific proteins than do homoglycopolymers, as a result of the heteromultivalent effect and binding ability between different sugar moieties and lectins.

 Please wait while we load your content...
Please wait while we load your content...