Synthesis of 19F nucleic acid–polymer conjugates as real-time MRI probes of biorecognition†
Abstract
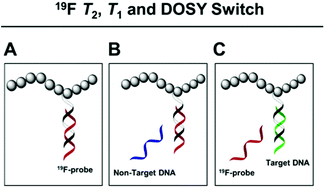
Polymer–DNA conjugates in which one nucleic acid strand contains fluorine-substituted nucleobases have been prepared and characterised. The efficacy of these novel 19F nucleic acid–polymer conjugates as sensitive and selective in vitro reporters of DNA binding events is demonstrated through a number of rapid-acquisition MR sequences. The conjugates respond readily and in a sequence specific manner to external target oligonucleotide sequences by changes in hybridisation. In turn, these structural changes in polymer–nucleotide conjugates translate into responses which are detectable in fluorine relaxation and diffusion switches, and which can be monitored by in vitro Spin Echo and DOSY NMR spectroscopy. Although complementary to conventional FRET methods, the excellent diagnostic properties of fluorine nuclei make this approach a versatile and sensitive probe of molecular structure and conformation in polymeric assemblies.


 Please wait while we load your content...
Please wait while we load your content...