The nature of the light absorption and emission transitions of 4-hydroxybenzophenone in different solvents. A combined computational and experimental study†
Abstract
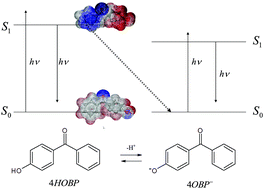
The photophysics and photochemistry of 4-hydroxybenzophenone (4HOBP) are interesting because they can give some insight into the behavior of humic material. Here we show that 4HOBP has a number of fluorescence peaks: (i) an intense one at excitation/emission wavelengths Ex/Em ∼ 200–230/280–370 nm, likely due to an excitation transition from S0 to S5 or S6, followed by S2 → S0 in emission (Sn denotes the singlet states of 4HOBP); (ii) a minor peak at Ex/Em ∼ 270–300/320–360 nm (S0 → S2 in absorption and S2 → S0 in emission), and (iii) very interesting signals in the typical emission region of humic substances, most notably at Ex/Em ∼ 200–220/400–500 nm and Ex/Em ∼ 260–280/400–470 nm (in both cases the emission corresponded to an S1 → S0 transition). The peak (i) (Ex/Em ∼ 200–230/280–370 nm) is quite intense at low 4HOBP concentration values, but it undergoes an effective inner-filter phenomenon. Remarkably, 4HOBP shows fluorescence peaks that arise from S2 → S0 transitions and that do not follow Kasha's rule. Fluorescence is observed in aprotic or poorly protic solvents, and to a lesser extent in aqueous solution. The excited states of 4HOBP, and most notably 4HOBP-S1, are much stronger acids than 4HOBP-S0. Therefore, excited 4HOBP is quickly deprotonated to 4OBP−-S0 in ∼neutral solution, with a considerable loss of the fluorescence properties. Higher fluorescence intensity can be observed under acidic conditions, where excited-state deprotonation is less effective, and in basic solution where the dissociated 4OBP−-S0 form prevails as the ground state. The excited states of 4OBP− are formed directly upon radiation absorption, and being weak bases they do not undergo important acid–base equilibria. Therefore, they can undergo radiational deactivation to produce significant fluorescence emission.


 Please wait while we load your content...
Please wait while we load your content...