Evaluation of O-alkyl and aryl sulfonyl aromatic and heteroaromatic amidoximes as novel potent DNA photo-cleavers†
Abstract
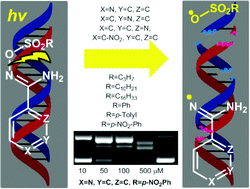
Several stable O-alkyl and aryl sulfonyl conjugated p-nitro-Ph and o-, m-, p-pyridine N′-hydroxy imidamides, were subjected to UV irradiation at 312 nm with supercoiled circular plasmid DNA pBluescript KS II. The generated amidinyl and sulfonyloxyl radicals led to effective DNA photo-cleavage. Both alkyl and aryl sulfonyl derivatives were active and the order p-pyridine > p-nitro-Ph > o-pyridine > m-pyridine was schematized for the N′-hydroxy imidamides moiety. Calf thymus-DNA affinity studies which comprised UV interactions, viscosity experiments and competitive studies with ethidium bromide showed good to excellent affinity of the compounds. These properties revealed sulfonyl amidoximes as novel effective DNA-photo-cleavers and may serve in the discovery of new leads for “on demand” biotechnological and medical applications.

 Please wait while we load your content...
Please wait while we load your content...