The kinetics and mechanism of photooxygenation of 4′-diethylamino-3-hydroxyflavone†
Abstract
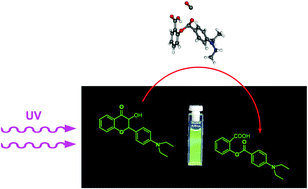
The photolysis reactions of 4′-diethylamino-3-hydroxyflavone (D), a versatile fluorescent probe showing excited-state intramolecular proton transfer (ESIPT), and the magnesium chelate of D (MgD2+) have been studied in acetonitrile solution. Upon UV irradiation both species were oxidized into O-4-diethylaminobenzoyl salicylic acid, differently from the photoreaction of the parent compound 3-hydroxyflavone (3HF) which was described to undergo rearrangement to 3-hydroxy-3-phenyl-indan-1,2-dione. The photooxygenation of the Mg2+ complex was found to be significantly faster than the reaction of the pure dye. As the kinetic analysis of the absorption spectra of samples under irradiation showed, the rate coefficients for the oxygenations of the excited state dye and complex have close values, kox(D*) = 2.4 × 107 min−1, kox(MgD2+*) = 3.9 × 107 min−1; the difference arises from the higher photooxygenation quantum yield of the complex, Φ(MgD2+) = 2.3 × 10−3, than the respective value for the pure dye, Φ(D) = 1.5 × 10−4. The potential energy surface of the photooxygenation of D was calculated assuming a reaction path in which the phototautomer formed from Dvia ESIPT, reacts in its triplet state with triplet molecular oxygen O2, a mechanism similar to that suggested for the photoreaction of the parent 3HF. The moderate values for the transition state energies confirmed the plausibility of the hypothetical mechanism.

 Please wait while we load your content...
Please wait while we load your content...