C–H activation enables a rapid structure–activity relationship study of arylcyclopropyl amines for potent and selective LSD1 inhibitors†
Abstract
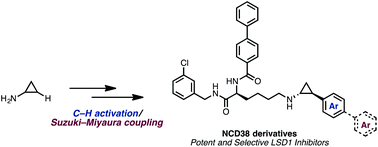
We describe the structure–activity relationship of various arylcyclopropylamines (ACPAs), which are potent LSD1 inhibitors. More than 45 ACPAs were synthesized rapidly by an unconventional method that we have recently developed, consisting of a C–H borylation and cross-coupling sequence starting from cyclopropylamine. We also generated NCD38 derivatives, which are known as LSD1 selective inhibitors, and discovered a more effective inhibitor compared to the original NCD38.


 Please wait while we load your content...
Please wait while we load your content...