Binding position analysis of target proteins with the use of amidopyrene probes as LA-LDI enhancing tags†
Abstract
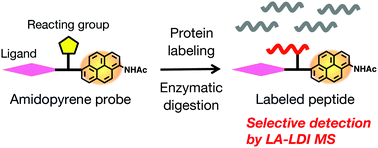
Amidopyrene-conjugated compounds can be detected by label-assisted laser desorption/ionization mass spectrometry (LA-LDI MS) without matrixes. When actin, a cytoskeletal protein, was labeled with an excess amount of amidopyrene N-hydroxysuccinate (apy–OSu), eight apy-labeled actin peptides were predominantly detected by LA-LDI MS. Then actin was labeled with an amidopyrene NHS ester of the antitumor marine macrolide aplyronine A (ApA–apy–OSu) to form a 1 : 1 conjugate. The sequence of an apy-labeled peptide was established as A108PLNPKANR116 by MS/MS analysis, in which the NHS ester moiety specifically reacted with the ε-amino group of K113. While the fragmentation at the linker part reduces the detection sensitivity of apy-labeled peptides on LA-LDI MS, our chemical probe method is useful for analyzing the binding modes of various ligands and target biomacromolecules that include multiple and weak interactions.

 Please wait while we load your content...
Please wait while we load your content...