Safe generation and use of bromine azide under continuous flow conditions – selective 1,2-bromoazidation of olefins†
Abstract
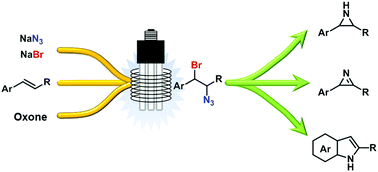
Bromine azide (BrN3), a useful but extremely toxic and explosive reagent for the preparation of vicinal 1,2-bromine azide compounds, was safely generated and reacted in situ with alkenes in a continuous flow photoreactor. BrN3 was generated by a novel procedure from NaBr and NaN3 in water, and efficiently extracted into an organic phase containing the alkene thus avoiding decomposition. The resulting addition products have been used for the preparation of several useful building blocks.

 Please wait while we load your content...
Please wait while we load your content...