OFox imidates as versatile glycosyl donors for chemical glycosylation†
Abstract
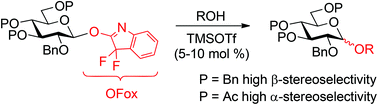
Previously we communicated 3,3-difluoroxindole (HOFox) – mediated glycosylations wherein 3,3-difluoro-3H-indol-2-yl (OFox) imidates were found to be key intermediates. Both the in situ synthesis from the corresponding glycosyl bromides and activation of the OFox imidates could be conducted in a regenerative fashion. Herein, we extend this study with the main focus on the synthesis of various OFox imidates and their investigation as glycosyl donors for chemical 1,2-cis and 1,2-trans glycosylation.

 Please wait while we load your content...
Please wait while we load your content...