Asymmetric syntheses of epohelmins A and B by In-mediated allylation†
Abstract
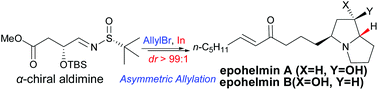
A diastereoselective new approach for the synthesis of trans-4-hydroxy-5-allyl-2-pyrrolidinone 9 has been developed through In-mediated allylation of α-chiral aldimine 8 with allyl bromide. The stereochemistry at the C-2 stereogenic center of 9 was controlled by both the α-OTBS substitution and the sulfinamide moiety. The utility of this asymmetric allylation is demonstrated by the asymmetric syntheses of epohelmins A (4) and B (10).

 Please wait while we load your content...
Please wait while we load your content...