Total synthesis, structural elucidation and anti-inflammatory activity evaluation of 2-deoxy-3,6-anhydro hexofuranoside derivatives isolated from Sauropus rostratus†
Abstract
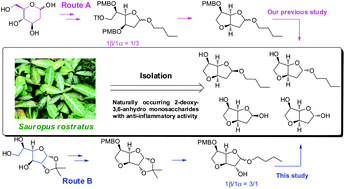
The first total synthesis of four 2-deoxy-3,6-anhydro hexofuranoside derivatives, namely sauropunols (A–D), isolated from the traditional Chinese medicinal plant Sauropus rostratus was accomplished. Structures of sauropunols A and B were clearly elucidated and reassigned. The anti-inflammatory activities of sauropunols (A–D) as well as the synthetic intermediates were evaluated, which is valuable for further structure–activity relationship (SAR) studies on this class of natural products.

 Please wait while we load your content...
Please wait while we load your content...