Flexible synthesis of cationic peptide–porphyrin derivatives for light-triggered drug delivery and photodynamic therapy†
Abstract
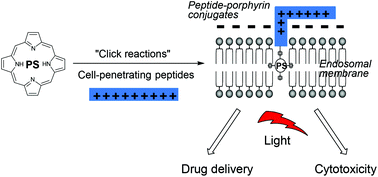
Efficient syntheses of cell-penetrating peptide–porphyrin conjugates are described using a variety of bioconjugation chemistries. This provides a flexible means to convert essentially hydrophobic tetrapyrolle photosensitisers into amphiphilic derivatives which are well-suited for use in light-triggered drug delivery by photochemical internalisation (PCI) and targeted photodynamic therapy (PDT).


 Please wait while we load your content...
Please wait while we load your content...