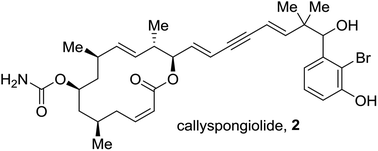
Enantioselective total synthesis and structural assignment of callyspongiolide†
Abstract
We have elucidated the complete absolute configuration of callyspongiolide and unambiguously assigned its stereochemistry at the C-21 center through synthesis. Four stereoisomers of callyspongiolide were synthesized in a convergent and enantioselective manner. A late-stage Sonogashira coupling forges the diene-ynic side chain. Other notable reactions are Yonemitsu's variation of Yamaguchi macrolactonization to cyclize an alkynic seco acid, highly trans-selective Julia–Kocienski olefination, CBS reduction to set the C-21 stereocenter, and methyl cuprate addition to an unsaturated pyranone to install the C-5 methyl center.

 Please wait while we load your content...
Please wait while we load your content...